When it comes to controlled substance compliance, meticulous record-keeping and adherence to regulations are paramount for veterinary professionals.
Balancing the intricacies of compliance and record keeping, alongside running a busy practice taking care of patients and clients, can certainly be a challenge for many. However, with a comprehensive understanding of the key elements involved, navigating this landscape becomes more manageable.
Jack Teitelman, CEO of Titan Group, former DEA Supervisory Agent, shares with us his common best practices that will help veterinary hospitals achieve a smoother and more effortless audit.
The dreaded DEA inspection looms over veterinary professionals, requiring them to present a multitude of documentation promptly. When it comes to institutions like methadone clinics and pharmacies, due to recent levels of scrutiny, their systems and processes, and effort invested to stay compliant are much more mature and battle tested. Veterinary professionals however may find themselves more worried about how they stack up during an audit.
To bridge this gap, the first crucial step is implementing a robust system for controlled drug storage. DEA emphasizes the need for secure storage that cannot be easily removed. Even a simple measure like bolting a safe onto a cabinet can ensure compliance.
The responsibility of securing controlled substances extends to effective key management. Understanding who has access to the drugs and when is vital for maintaining accountability. Avoiding conspicuous signage or mislabeling cabinets is crucial, as DEA inspections meticulously scrutinize these aspects.
While the DEA mandates a biennial inventory, the real effort needed to be ever ready for an audit, lies in regular reconciliations. Precise and frequent counts of each drug, whether through visual inspection, weighing, or extraction and measurement, play a pivotal role in detecting and addressing discrepancies.

Within the domain of injectable medications, the DEA pays particular attention to ‘overfill’ or variance. Monitoring this overfill is crucial, as it can open avenues for theft or adulteration. A thorough understanding of the overfill variance specific to each drug is essential for effective inventory management and successful DEA inspections.
But how can veterinary professionals streamline these essential tasks without compromising their primary role of caring for animals?
This is where technology comes to the rescue. VetSnap, a powerful software solution tailored for veterinary professionals, simplifies the compliance process and reduces the burden.
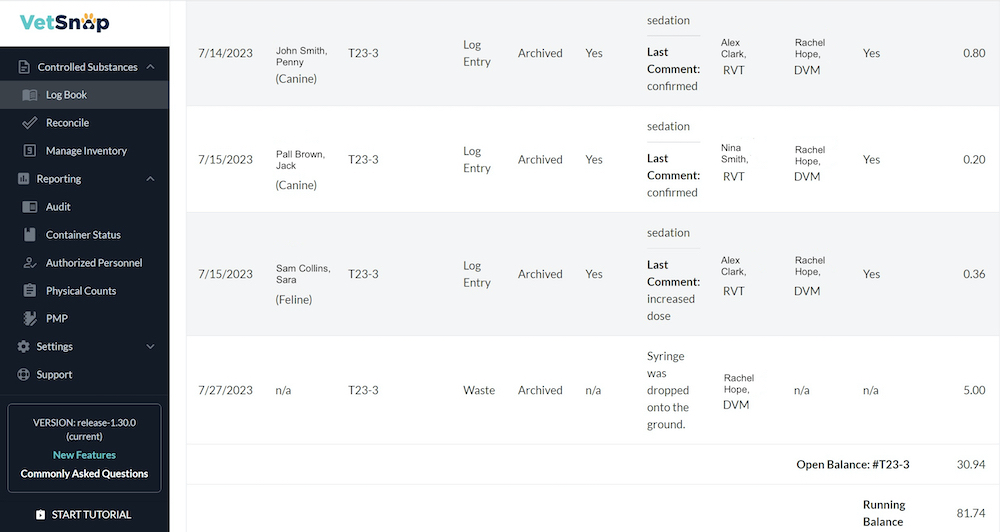
The software incorporates advanced tracking mechanisms that ensure precise reconciliation of drug inventories. The ability to help you reconcile by weight and volume significantly reduces the risk of inaccuracies and variances in drug counts. VetSnap also streamlines managing and tracking unopened and opened containers, ensuring that every drug is accounted for and discrepancies are flagged in real-time.
Cecilia Baer, VetSnap’s Sales Director, explains how exactly VetSnap helps your hospital’s controlled substance compliance process become easier and more accurate. “Logs are simple to create with a level of detail that would take many minutes by hand, but less than 30 seconds with auto-populated details that are less prone to manual entry error. Reconciliation is sped up significantly with easy daily validation between the logbook and the PIMS. Signatures and permission levels allow an extra degree of control and auditability so more people that should be involved in the logging process, can be involved. Finally, by using the same reports that DEA agents are familiar with such as our Day-by-Day Accountability Audit Report, you can be sure that you’re ready for the knock whenever it happens.”

